Translate this page into:
Antiproliferative Activity Extracts of Simarouba glauca and Euphorbia hirta Against Colorectal Cancer Cells
*Corresponding author: Kanchan K. Mishra, Stem Cells and Regenerative Medicine, Surat Raktadan Kendra and Research Centre, Surat, Gujarat, India. kanchan008@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Prajapati C, Patel P, Reddy MN, Mishra KK. Antiproliferative Activity Extracts of Simarouba Glauca and Euphorbia Hirta Against Colorectal Cancer Cells. Glob J Med Stud. doi: 10.25259/GJMS_23_2024
Abstract
Objectives:
The aim of the present study was to study the antiproliferative, apoptosis and gene expression level of an extracts derived from the medicinal plants Simarouba glauca and Euphorbia hirta on colorectal cell lines.
Material and Methods:
In this study, the fresh leaves of S. glauca and whole plant of E. hirta were subjected to solvent extraction using ethanol. The plant extracts were tested for the anticancer activity by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) assay. MTT assay was performed on HCT-116 (human colon colorectal carcinoma) and Caco-2 (human colon colorectal adenocarcinoma) cell lines. Deoxyribonucleic acid (DNA) fragmentation and real-time polymerase chain reaction assay were performed to check apoptosis and gene expression level.
Results:
The ethanolic extracts of S. glauca and E. hirta exhibited significant antiproliferative activity against colorectal cancer (CRC) cells – HCT-116 and CaCo2. Cytotoxicity activity of plant extracts on HCT-116 was more compared to CaCo2 cells. E. hirta shows more cytotoxicity activity compared to S. glauca extracts. Cell exposed at 160 and 320 μg/mL concentrations of plant extracts shows fragmentation of DNA. Increased expression of myelocytomatosis oncogene (MYC) gene and reduced expression of Cyclin D1 (CCND1) and Baculoviral IAP Repeat Containing 5 (BIRC5) gene show evidence that the extracts of S. glauca and E. hirta might induce apoptosis. This result shows that the extracts of S. glauca and E. hirta possessed immense potential treatment of CRC.
Conclusion:
Ethanolic extracts of Simarouba glauca and Euphorbia hirta exhibit strong antiproliferative activity against colorectal cancer, inducing apoptosis and demonstrating promising therapeutic potential for further exploration.
Keywords
Antiproliferative activity
Deoxyribonucleic acid fragmentation
Euphorbia hirta plant
HCT-116
Simarouba glauca plant
INTRODUCTION
Cancer is a group of diseases that promote the growth of abnormal cells which has ability to invade or spread to other parts of the body and this abnormal growth cannot be controlled or stopped.1 The third most common cancer in men and women is colorectal cancer (CRC). The major risk of developing CRC is some lifestyle factors such as consuming processed meat, alcohol drinks, red meat and being overweight.2 CRC is higher in developed countries than in undeveloped nations and adults age more than 50 years are more prone to have CRC mainly in males than in females.3 Some genetic errors are responsible for affecting the control of apoptosis which lead to increase in CRC.4 This leads to affect apoptosis process and its related pathway. Therefore, triggering apoptosis is one of the major objectives for the prevention of cancer.5 Modern treatments such as chemotherapy, surgical and radiations cause various side effects. Nowadays, there is a need to develop drugs which has less toxicity and less side effects.6 Hence, scientists are focusing on Indian traditional system of medicine, which are natural and safe. Phytochemicals played an important role for the treatment of cancer.7 In cancer treatment, majority of drugs are isolated from medicinal plants.8
Simarouba glauca is a polygamo-dioecious multipurpose evergreen tree and belongs to family Simaroubaceae and commonly known as ‘The Paradise Tree’ or ‘King Oil Seed Tree’ or ‘Laxmitaru Tree’ with the height of 7–15 m.9 It possesses various types of medicinal and pharmacological properties. Some bioactivities such as haemostatic, anthelmintic, antiparasitic, antidysenteric, antipyretic and anticancerous were shown by the bark and leaf extract of S. glauca.10 Analgesic, antimicrobial, antiviral, astringent, emmenagogue, stomachic, tonic and vermifuge were reported from the leaf, fruit, pulp and seed of S. glauca.11 Isolation of four alkaloids derivatives from S. glauca shows cytotoxic activity against various human cancer cells (colon, oral, prostate and lung).12 Canthin-6-one isolated from Picrasma quassioides possesses cytotoxic activity against nasopharynx carcinoma cells.13 Some quassinoids – glaucarubin, glaucarubinone, glaucarubol and glaucarubolone – possess anticancer activity against KB cells.14 The chloroform soluble extract of S. glauca shows good cytotoxic activity against several human cancer cell lines.15
Euphorbia hirta is used in respiratory disorders (asthma, cough and bronchitis), dysentery, jaundice, tumours and various female disorders and it is widely used as traditional herbal medicine.16 In India, it is available on open grasslands, roadsides and pathways. The extracts of E. hirta are used in the treatment of various diseases such as dysentery, diarrhoea, asthma and hay fever. Plant extracts used as an analgesic, antipyretic and anti-inflammatory activities.17 It also shows anticancer activity, antifungal, antibacterial and nematicidal activity. To cure eyesores, white milky latex of plant can be used.17 It is also used in skin diseases, snake bites,18 antiamoebic,19 antispasmodic,20 antimalarial, antiasthmatic and antioxidant.16
In the present study, in vitro anticancer activity was analysed on CRC cell lines – HCT-116 and Caco2 by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) assay from leaf extract of S. glauca and whole plant of E. hirta and also described the molecular mode of action in many concentrations of treated cells from the expression of several Wnt signalling pathway genes that were responsible for programmed cell death.
MATERIAL AND METHODS
Collection of plant materials
Fresh leaves of S. glauca were collected from Sardarkrushinagar Dantiwada Agricultural University, Dantiwada, Banaskantha, Gujarat, India, and whole plant of E. hirta was collected from Shree Bapalal Vaidya Botanical Research Centre, located in Veer Narmad South Gujarat University Campus, Udhna Magdalla Road, Surat, Gujarat, India; both the plant materials were washed under the running tap water and dried at 45°C in the oven. The dried materials were then homogenised to fine powder and stored in air tight container for further use.
Extraction of plant materials
For extraction, approximately 5 g of dried powder were taken in 50 mL of ethanol (70%) and kept under gentle and continuous shaking on an orbital shaker (Orbital Shaking Incubator - Rajendra Electric Motor Industries [REMI]) for 24 h. The liquid was clarified by filtration using Whatman No.1 papers to obtain the crude extracts and further concentrated. The dry weight of the extracts were obtained by the solvent evaporation and used to determine concentration.
Culture medium and cell lines
HCT-116 (human colon colorectal carcinoma) and Caco-2 (human colon colorectal adenocarcinoma) cell lines were purchased from National Centre for Cell Science, Pune. Culture of all cell lines was done in tissue culture flasks with advanced Dulbecco’s Modified Eagle Medium (DMEM) containing 100 U/mL penicillin, 10% heat-inactivated foetal bovine serum and 100 μg/mL streptomycin solutions and incubated at 37°C with 5% carbon dioxide (CO2). Cell culture media and reagents were purchased from Gibco Company (Germany).
Cytotoxicity study
Antiproliferative activity of crude extracts was carried out by MTT assay.21 In MTT assay, only viable tumour cells reduce yellow soluble MTT into purple (blue) insoluble formazan products, which may be measured spectrophotometrically.22
Briefly, 200 μL of cells (1 × 106 cells/mL) were seeded in 96 well flat bottom microtiter plates and incubated at 24 h (37°C, 5% CO2). After 24 h, various concentrations of plants sample (10–320 μg/mL) were incubated for 24 h at similar conditions. After 24 h of incubation, 20 μL of MTT solution (5 mg/mL in phosphate buffer solution) was added to each well and kept the plate for another 3 h at 37°C. To dissolve formazan crystals formed, medium containing MTT was gently replaced by dimethyl sulfoxide (DMSO) and stirred for 10 min at room temperature. Absorbance was measured at 590 nm using spectrophotometric microplate reader (Epoch Elisa reader). Standard drug cisplatin was used as positive control. The assays were performed in triplicate. The effect of the samples on the proliferation of HCT-116 and Caco2 cells was expressed as the % cell viability. Then, % cell viability of plant extracts was calculated using the formula, Cytotoxicity (%) = Optical Density (OD) of control sample – OD of treated sample/OD control sample × 100.
Deoxyribonucleic acid (DNA) fragmentation assay (apoptosis)
DNA fragmentation assay was used to determine the induction of cell death in treated HCT-116 cells. HCT-116 cells (1 × 106 per mL) were treated with extracts of S. glauca and E. hirta at concentrations 160 and 320 μg/mL and control cells were untreated cells for 48 h. The cells were washed twice with phosphate buffer solution after stimulation. Then, DNA extraction of treated cells was done by phenol/chloroform method. DNA samples were electrophoretically separated on 1.5% agarose gel in 1X Tris-acetate-ethylenediaminetetraacetic acid buffer. DNA fragmentation was observed under ultraviolet transilluminator.
Quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) assay
Real-time RT-PCR was carried out in 7500 real-time polymerase chain reaction (PCR) system (Applied Biosystems). The expression levels of genes are MYC, BIRC5 and CCND1. b -Actin is used as housekeeping gene. HCT-116 cells (1 × 106 per mL) were treated with extracts of S. glauca and E. hirta at concentrations 160 and 320 μg/mL and control cells were untreated cells for 48 h. The protocol consisted of a denaturing cycle at 95°C for 10 min followed by 40 cycles of 10s at 95°C, 30s at 60°C and 30s at 72°C. The reaction setup resumed in Table 1 and the sequence of primers used was listed in Table 2. Expression levels of the analysed genes were normalised to the housekeeping gene (b-actin) and correlated to the untreated control cells group using the ΔΔCt method.23
| Components | 25 µL reaction |
|---|---|
| Primer Forward | 1.0 |
| Primer Reverse | 1.0 |
| Template DNA | 2.5 |
| Water | 8 |
| Syber green (Master Mix) | 12.5 |
PCR: Polymerase Chain Reaction
| Gene | Primer pair | Sequence | Tm | Annealing temperature for PCR amplification | Product size (bp) |
|---|---|---|---|---|---|
| β– Actin | FP | TCCTCCTGAGCGCAAGTACTCT | 66.4 | 55 | 153 |
| RP | GCTCAGTAACAGTCCGCCTAGAA | 65.5 | |||
| MYC | FP | TAGTGGAAAACCAGCAGCCT | 57.3 | 47 | 701 |
| RP | GAGCAGAGAATCCGAGGACG | 61.4 | |||
| BIRC5 | FP | TGAGAACGAGCCAGACTTGG | 59.4 | 50 | 87 |
| RP | TGTTCCTCTATGGGGTCGTCA | 59.8 | |||
| CCNDI | FP | AGCTGTGCATCTACACCGAC | 59.4 | 49 | 113 |
| RP | GAAATCGTGCGGGGTCATTG | 59.4 |
PCR: Polymerase Chain Reaction, FP: Forward Primer, RP: Reverse Primer, Tm: Temperature.
Statistical analyses
All experiments were performed in triplicates and the data were presented as mean ± Standard deviation. Microsoft Excel 2007 was used for graphical representation. Probability values P < 0.05 were considered statistically significant.
RESULTS
Cytotoxicity activity was evaluated against HCT-116 and Caco2 cells by crude ethanolic (70%) extracts of S. glauca and E. hirta using the MTT assay. The graphical representation of inhibition (Y-axis) versus concentration (X-axis) indicates significant reduction in the viability of HCT-116 and Caco2 cells. Crude ethanolic extract was tested at concentrations of 10, 20, 40, 80, 160 and 320 μg/mL. The Inhibitory concentration 50 (IC50) values of S. glauca and E. hirta were determined as 102.48 and 79.29 μg/mL for HCT-116 and 123.21 and 110.05 μg/mL for Caco2 cells, respectively. Cytotoxicity activity of the extracts of S. glauca and E. hirta on HCT-116 was more compared to the Caco2 cells. E. hirta shows higher cytotoxic activity compared to S. glauca extracts. The results show that the activity of the extracts reveals that they are dose dependent as shown in Figure 1.
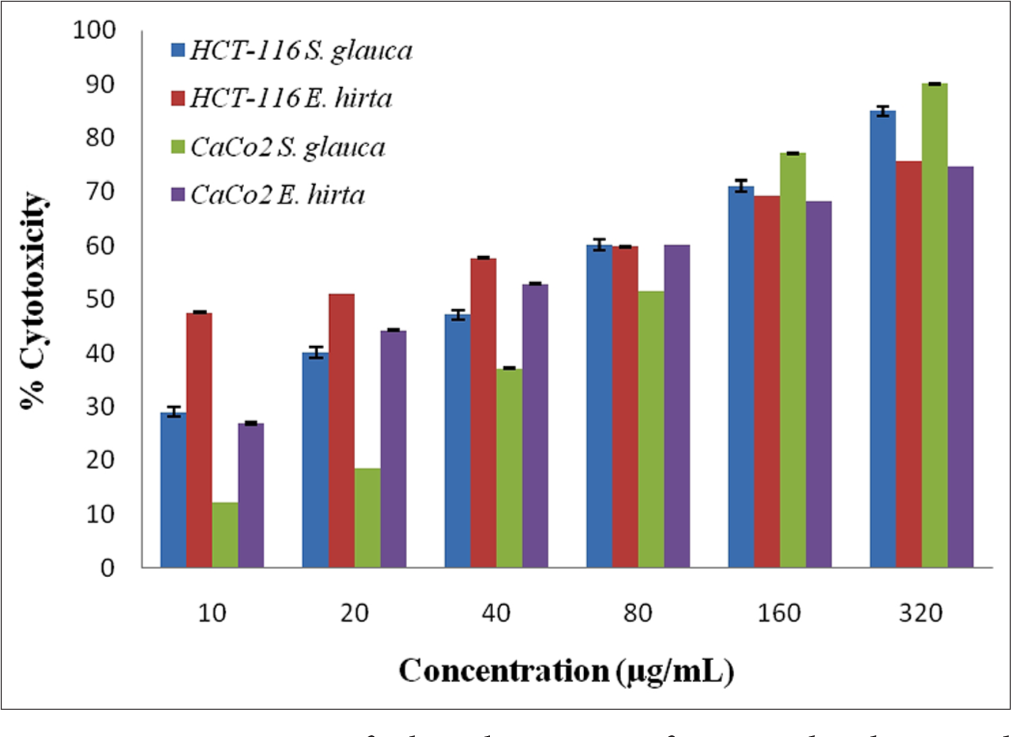
- Cytotoxicity of ethanolic extract of Simarouba glauca and Euphorbia hirta against HCT-116 and CaCo2 cells.
For DNA fragmentation, DNA is extracted from the apoptotic cells and separated in an agarose gel. The potency of S. glauca and E. hirta extracts to induce DNA fragmentation in human CRC cell HCT-116 was tested. The HCT-116 cells treated with two plant extracts (S. glauca and E. hirta) indicated the presence of DNA fragmentation, which confirmed the antiproliferative effect of plant, extracts [Figures 2 and 3].
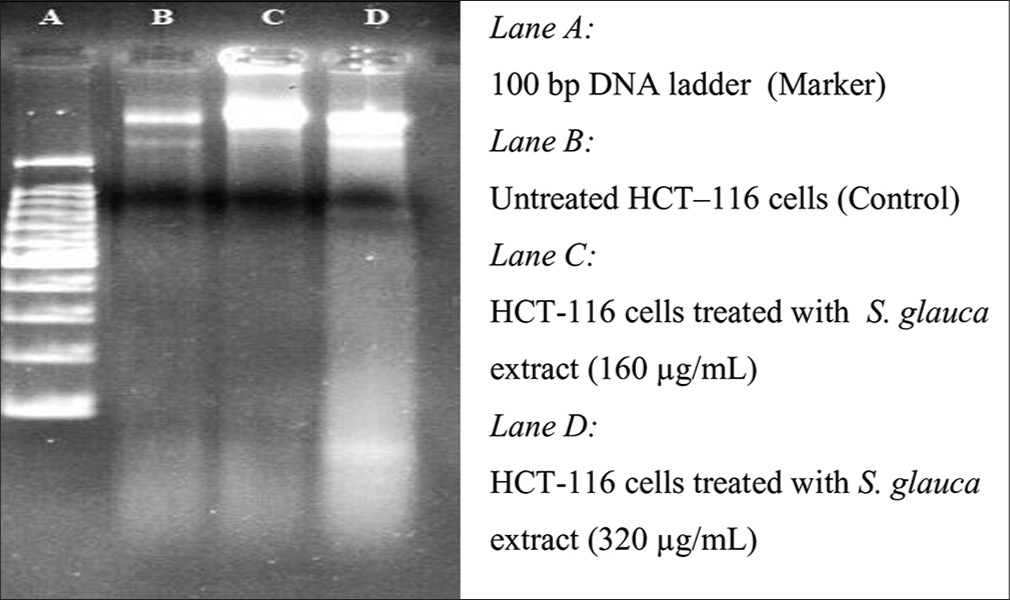
- Agarose gel electrophoresis of the chromosomal deoxyribonucleic acid extracted of HCT-116 cells treated with Simarouba glauca extract at different concentration for 48 h.
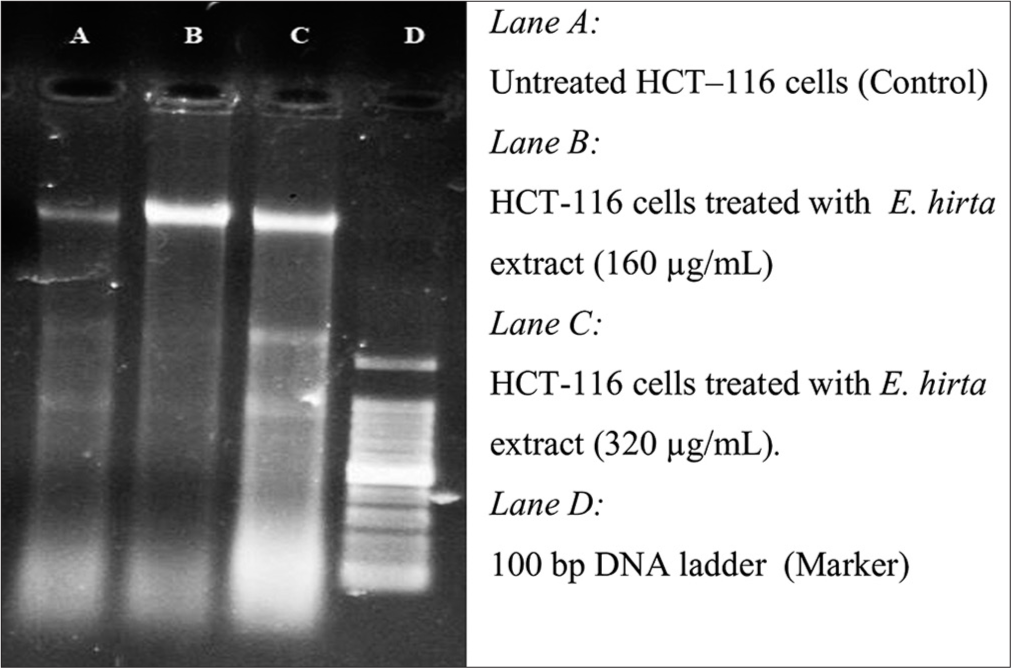
- Agarose gel electrophoresis of the chromosomal deoxyribonucleic acid extracted of HCT-116 cells treated with Euphorbia hirta extract at different concentration for 48 h.
The HCT-116 cells treated with two plant extracts (S. glauca and E. hirta) indicated the presence of DNA fragmentation, which confirmed the antiproliferative effect of plant extract. DNA of the treated cells resulted in smaller fragments, which are considered as the trademark of treated cells undergoing apoptosis.24
From [Figures 4-7], effect of S. glauca and E. hirta on gene expression of MYC, BIRC5 and CCND1 was studied in HCT-116 cells by real-time PCR. The expression levels of some Wnt signalling pathway genes such as BIRC5, CCND1 and MYC were examined to understand the molecular mechanism in treated HCT-116 cells. The internal control b-actin was used as a control for gene expression. S. glauca and E. hirta extracts suppressed the CCND1 in a dose-dependent manner by 0.18 and 0.08 fold in S. glauca; 1.93 and 0.67 folds in E. hirta at 160 and 320 μg/mL, respectively, whereas 0.93 and 0.55 fold in S. glauca; 2.47 and 0.68 folds in E. hirta at 160 and 320 μg/mL, respectively, were found in BIRC5 mRNA expression study. For MYC, expression is 0.03 and 0.04 fold in S. glauca whereas 0.54 and 0.74 fold in E. hirta at 160 and 320 μg/mL, respectively. Expression of MYC was found to be upregulated at higher concentration in S. glauca and E. hirta samples in HCT-116 cells.
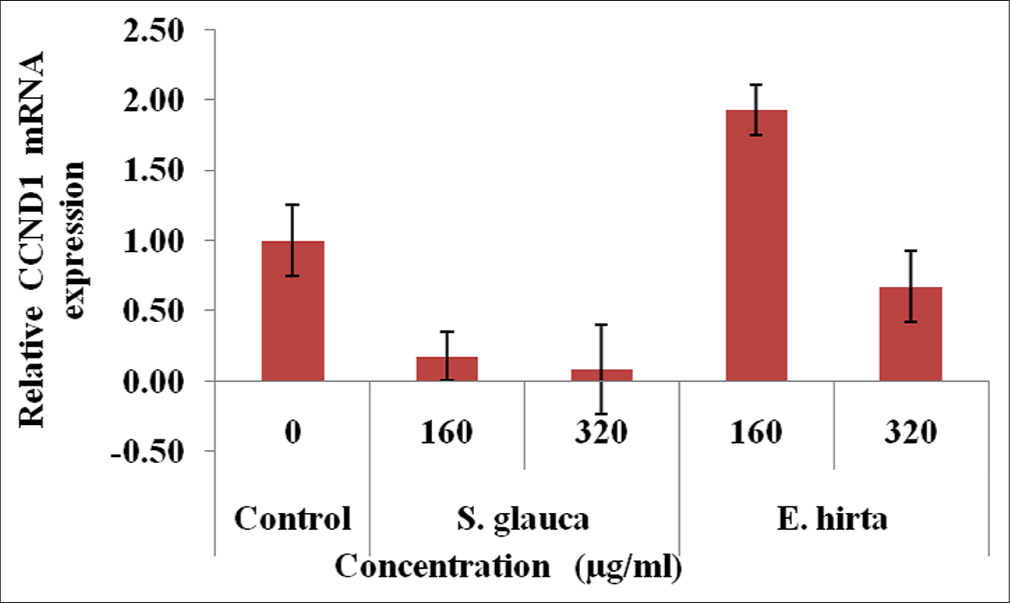
- Fold difference in CCND1 gene relative to untreated HCT-116 cells = 2−ΔΔCt.
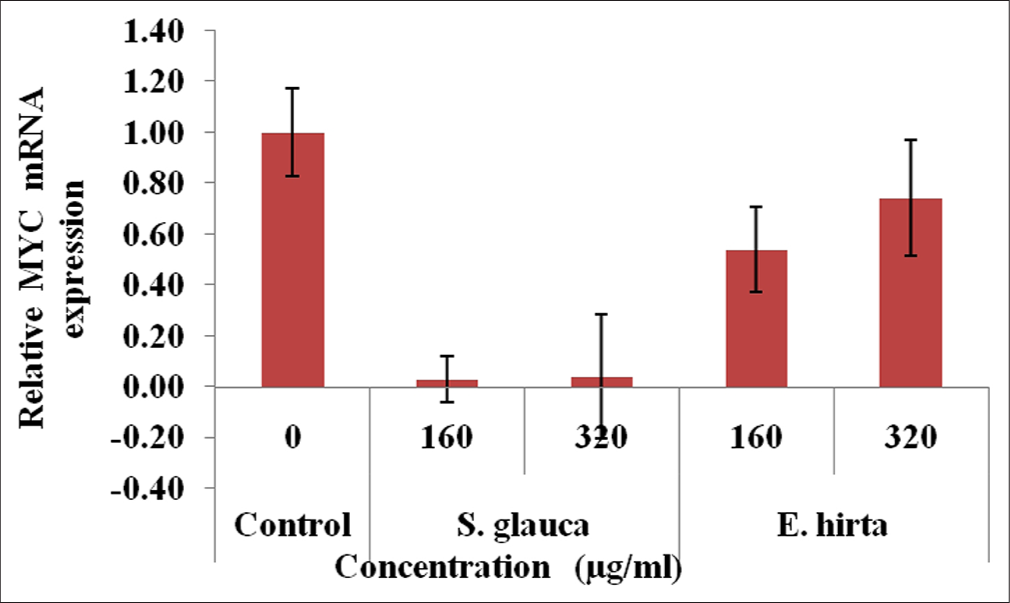
- Fold difference in MYC gene relative to untreated HCT-116 cells = 2−ΔΔCt.
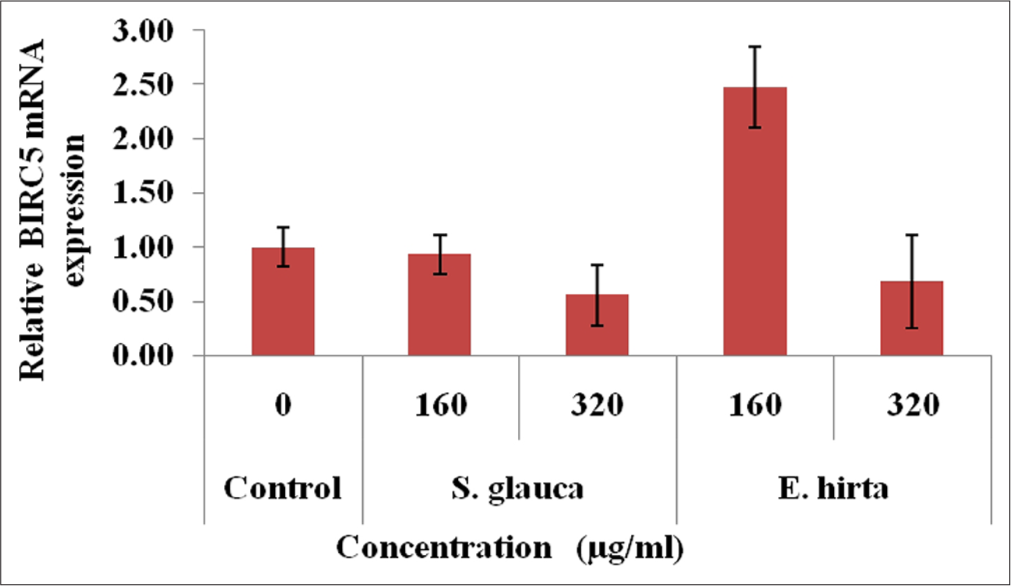
- Fold difference in BIRC5 gene relative to untreated HCT-116 cells = 2−ΔΔCt.
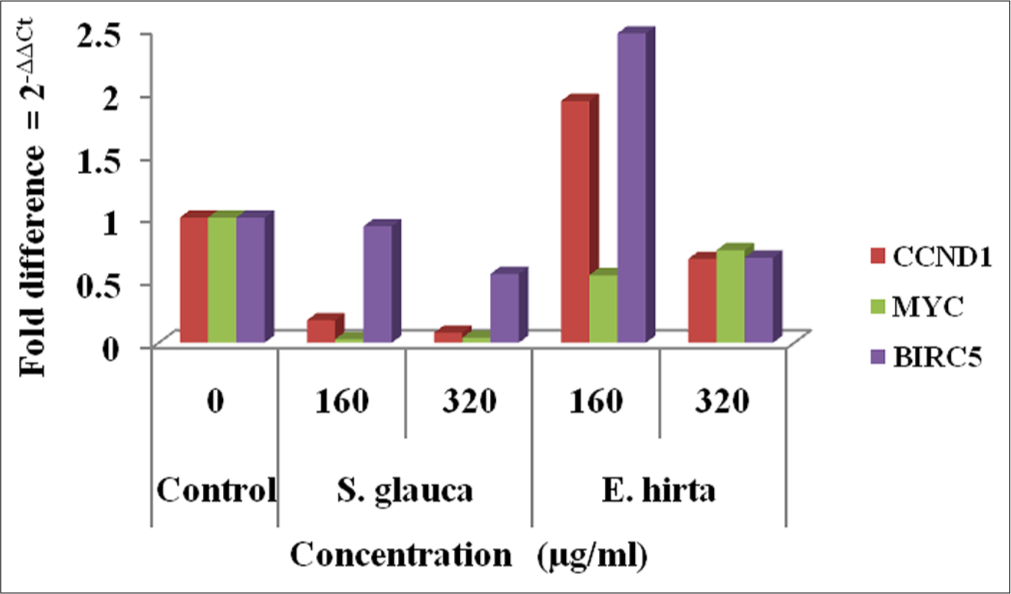
- Graphical representation of expression levels of CCND1, MYC and BIRC5 after Simarouba glauca and Euphorbia hirta treatment.
DISCUSSION
The growth-inhibitory effect of 70% ethanolic extracts of S. glauca and E. hirta was examined on two human adherent cancer cell lines HCT-116 and Caco-2 using untreated cells as negative controls. The results of these experiments are shown in Figures 1. As can be seen, the extracts of S. glauca and E. hirta showed good anticancer activity towards two cell lines. Results are in dose dependent which shows decrease in cell viability with increase in extracts concentration. The results showed that S. glauca and E. hirta extracts effectively inhibited the growth of HCT-116 and Caco-2 in a dose-dependent manner, with an IC50 value 102.48 and 79.29 μg/mL and 123.21 and 110.05 μg/mL, respectively. From the data, it is said that S. glauca and E. hirta extracts have the potential anti-cancer effect towards CRC cell lines. The study finding is in agreement with Jose et al.25 They found that S. glauca extract showed a potential anticancer activity against various cell lines by in vitro approach. Furthermore, in our previous study, the cytotoxic effect of methanolic extract of S. glauca against leukaemic cancer cell lines (K-562, T lymphoblast cell line (MOLT-3) and human acute myelogenous leukemia [KG-1]) was reported by Prajapati et al.26 Consistent with this study finding, the various extracts base on increasing polarity of S. glauca leaves showed cytotoxicity in T-24 bladder cancer cell line.27 According to Anitha et al.,28 ethanolic extract of E. hirta gives potential anticancer activity on Dalton’s lymphoma ascites cells and Ehrlich ascites tumour cells. Antitumor activity was reported using methanolic extract of leaves of E. hirta and gives most effective inhibition of HEp-2 cell proliferation.29 Furthermore, in vitro anticancer activity of ethanolic extract of E. hirta on myeloid leukaemia cell line (HL-60) was reported by Sharma et al.30
In the present study, expression levels of 3 target genes (BIRC5, CCND1 and MYC) and an endogenous control (b-actin) are evaluated. The results shown in Figure 7 indicate that S. glauca and E. hirta extracts suppressed the CCND1 in a dose-dependent manner by 0.18 and 0.08 fold in S. glauca; 1.93 and 0.67 fold in E. hirta at 160 and 320 μg/mL, respectively, whereas 0.93 and 0.55 fold in S. glauca; 2.47 and 0.68 fold in E. hirta at 160 and 320 μg/ml, respectively, were found in BIRC5 mRNA expression study. Expression of MYC was found to be upregulated at higher concentration in S. glauca and E. hirta samples in HCT-116 cells.
The potential anticancer compounds have ability to kill cancer cells without creating any side effect to normal cells. These types of condition can be possible by causing apoptosis. Under apoptosis condition, cell shows some types of morphological changes such as DNA fragmentation and cell shrinkage. The potency of S. glauca and E. hirta extracts to induce DNA fragmentation in human CRC cell HCT-116 was tested. The HCT-116 cells treated with two plant extracts (S. glauca and E. hirta) indicated the presence of DNA fragmentation which confirmed antiproliferative effect of plant extracts [Figures 2 and 3]. DNA of the treated cells resulted into smaller fragments which are considered as trademark of treated cells undergoing apoptosis. DNA fragmentation in the plant extracts treated cells was confirmed using agarose gel electrophoresis, which shows the presence of DNA ladder (a marker of apoptosis), in the extracts treated HCT-116 cells. However, the untreated control cells show no evidence of DNA fragmentation. As shown in [Figures 2 and 3], the treated cells show increased DNA fragmentation in a dose-dependent manner.
The study revealed that the anticancer plants extracts of S. glauca and E. hirta have greater potential for employing them as strong agents to control CRC cells.
CONCLUSION
The findings of this study demonstrate that ethanolic extracts of Simarouba glauca and Euphorbia hirta exhibit significant antiproliferative activity against colorectal cancer cell lines, with E. hirta showing higher cytotoxicity compared to S. glauca. The observed DNA fragmentation and gene expression analysis suggest that these extracts may induce apoptosis by increasing the expression of the MYC gene and decreasing the expression of CCND1 and BIRC5. These results highlight the potential of S. glauca and E. hirta as promising natural candidates for the development of therapeutic agents targeting colorectal cancer. Further in-depth studies are warranted to elucidate their mechanisms of action and validate their efficacy in vivo.
Ethical approval:
Ethical approval was obtained from the Institutional Ethics Committee (IEC) of Surat Raktadan Kendra & Research Centre. Approval Reference Number: SRKRC/RP/14/2017. Date: 25th February, 2017.
Declaration of patient consent:
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: This is intramural project supported by the our institute.
References
- Evaluation of in Vitro Anticancer Activity of Symplocos cochinchinensis (Lour.) S. Moore Bark. Int J Herb Med. 2016;4:117-9.
- [Google Scholar]
- “Diet, Nutrition, Physical Activity and Colorectal Cancer. 2018. Available from: https://www.wcrf.org/dietandcancer [Last accessed on 2024 Sep 12]
- [Google Scholar]
- Polyphenols in Colorectal Cancer: Current State of Knowledge Including Clinical Trials and Molecular Mechanism of Action. Biomed Res Int. 2018;2018:4154185.
- [CrossRef] [PubMed] [Google Scholar]
- An Overview of Apoptosis and the Prevention of Colorectal Cancer. Crit Rev Oncol Hematol. 2006;57:107-21.
- [CrossRef] [PubMed] [Google Scholar]
- Cytotoxic and Apoptotic Activities of Amorphophallus campanulatus (Roxb.) Bl. Tuber Extracts against Human Colon Carcinoma Cell Line HCT-15. Saudi J Biol Sci. 2014;21:524-31.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of Anticancer Activity Using Hexanic Extract of Vitex trifolia on Two Different Cancer Cell Lines. Int J Pharmacogn Phytochem Res. 2014;6:449-53.
- [Google Scholar]
- Complementary and Alternative Medicine (CAM) in Mexican Patients with Cancer. Clin Transl Oncol. 2006;8:200-7.
- [CrossRef] [PubMed] [Google Scholar]
- Natural Products as Leads to Anticancer Drugs. Clin Transl Oncol. 2007;9:767-76.
- [CrossRef] [PubMed] [Google Scholar]
- A Critical Review on Medicinally Important Oil Yielding Plant Laxmitaru (Simarouba glauca DC.) India J Pharm Sci Res. 2011;3:1195-213.
- [Google Scholar]
- Oil Tree-Laxmitaru Simarouba glauca In: University of Agricultural Sciences. New Delhi, India: Bangalore and Indian Council of Agricultural Research; 2002. p. :86.
- [Google Scholar]
- Cytotoxic Constituents of the Twigs of Simarouba glauca Collected from a Plot in Southern Florida. Phytoter Res. 2005;19:136-40.
- [CrossRef] [PubMed] [Google Scholar]
- Canthin-6-one Alkaloids from Picrasma quassioides and their Cytotoxic Activity. J Asian Nat Prod Res. 2008;10:1009-12.
- [CrossRef] [PubMed] [Google Scholar]
- The Isolation and Structure of 13,18-Dehydroglaucarubinone a New Antineoplastic Quassinoid from Simarouba amara. Experientia. 1978;34:1122-3.
- [CrossRef] [PubMed] [Google Scholar]
- Cytotoxic and Antimalarial Alkaloids from the Tubers of Stephania pierrei. J Nat Prod. 1993;56:30-5.
- [CrossRef] [Google Scholar]
- Piper betle Linn. A Maligned Pan-Asiatic Plant with an Array of Pharmacological Activities and Prospects for Drug Discovery. Curr Sci. 2010;99:922-32.
- [Google Scholar]
- The Wealth of INDIA Raw Materials. Vol Vol 3. New Delhi: Council of Scientific and Industrial Research Publication; 1992. p. :270-1.
- [Google Scholar]
- Antiamoebic and Spasmolytic Activities of Extracts from Some Antidiarrhoeal Traditional Preparations used in Kinshasa, Congo. Phytomedicine. 2000;7:31-8.
- [CrossRef] [PubMed] [Google Scholar]
- Distribution Pattern of n-alkanes in Chilean Species from the Euphorbiaceae Family. Bol Soc Chil Quim. 1996;41:229-33.
- [Google Scholar]
- Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J Immunol Methods. 1983;65:55-63.
- [CrossRef] [PubMed] [Google Scholar]
- Optimization of the Tetrazolium Dye (MTT) Colorimetric Assay for Cellular Growth and Viability. Methods Mol Biol. 2011;716:157-68.
- [CrossRef] [PubMed] [Google Scholar]
- A New Mathematical Model for Relative Quantification in Real-time RT-PCR. Nucl Acids Res. 2001;29:e45.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor Cell Selective Cytotoxicity and Apoptosis Induction by an Herbal Preparation from Brucea javanica. N Am J Med Sci (Boston). 2011;4:62-6.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic Potential of Phytochemicals Isolated from Simarouba glauca for Inhibiting Cancers: A Review. Syst Rev Pharm. 2019;10:73-80.
- [CrossRef] [Google Scholar]
- Evaluation of Anticancer Activity using Leaf Extract of Simarouba glauca on Leukemic Cancer Cell Lines. Int J Bot Stud. 2018;3:52-6.
- [Google Scholar]
- Evaluation of in Vitro Antioxidant and Anticancer Activity of Simarouba glauca Leaf Extracts on T-24 Bladder Cancer Cell Line. Pharmacogn J. 2017;9:906-12.
- [CrossRef] [Google Scholar]
- In Vitro Anticancer Activity of Ethanolic Extract of Euphorbia hirta (L.) Sci Technol Arts Res J. 2014;3:8-13.
- [CrossRef] [Google Scholar]
- An in Vitro Study of Cytotoxic Activity of Euphorbia hirta on HEp-2 Cells of Human Epithelioma of Larynx. Int J Pharm Pharm Sci. 2011;3:101-3.
- [Google Scholar]
- Evaluation of the Antioxidant, Anti-Inflammatory, and Anticancer Activities of Euphorbia hirta Ethanolic Extract. Molecules. 2014;19:14567-81.
- [CrossRef] [PubMed] [Google Scholar]






